Alcohol: Drink, Chemical, Medicine, Poison
What Is Alcohol?
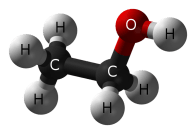
When you mention “alcohol”, you most certainly mean “ethanol”, a simple chemical which is created when grains, fruits, or vegetables are fermented. Fermentation is a process that takes place when yeast (fungi) convert food sugars into ethanol and carbon dioxide.
Sugar + yeast —> alcohol + carbon dioxide
Or
C6H12O6 + yeast ——> CH3CH2OH + CO2
Drink
 So what happens in your body when you start sipping this delicious martini????
So what happens in your body when you start sipping this delicious martini????
- Alcohol travels to your stomach.
- From the stomach, it is quickly passed into the bloodstream, where it travels in a few minutes to every part of the body, including your brain.
- In the brain alcohol affects your neurons: It alters neuron’s membranes and ion channels, enzymes such as acetylcholine, serotonin, and GABA receptors.
- When alcohol binds to these enzymes, it slows the function of the central nervous system and that’s why you feel more relaxed and stress-free.
- Ethanol also inhibits the production of a hormone (ADH) that regulates urine flow, causing increased urine production and dehydration.
- Alcohol increases dopamine by stopping enzymes that degrade it – that’s why you feel happier!
- Ethanol also causes blood vessels to dilate, resulting in flushing of the skin and a sensation of warmth as blood moves into capillaries.
- If you’ve had a large meal, alcohol will stay longer in your stomach, that’s why you can’t get drunk as easily. But eventually, it will enter the bloodstream and do its tricks:)
Your body sees alcohol as an intruder and tries to get rid of it using an enzyme to break it down, “alcohol dehydrogenase“. On contact, the enzyme snatches a hydrogen atom off the ethanol molecules in your drink, rendering it into non-intoxicating acetaldehyde, which is associated with hangovers. Alcohol dehydrogenase is less efficient in Asian populations. Lungs and liver also remove 10% of the alcohol in your body by urine and breath.
Click on this link for a schematic representation of how alcohol works in your brain.
Chemical
- Fuel: The largest single use of ethanol is as a motor fuel, fuel additive, and as a rocket fuel. Over 20% of cars in Brazil are able to run on 100% ethanol fuel.
- Raw material: Ethanol is an important industrial ingredient and has widespread use as a base chemical for other organic compounds, such as ethyl halides, ethyl esters, diethyl ether, acetic acid.
- Solvent: Ethanol is miscible with water and is a good general purpose solvent. It is found in paints, tinctures, markers, and personal care products such as perfumes and deodorants.
- Preservative: for biological specimens.
Medicine
- Antiseptic: Ethanol is used in antiseptic and some antibacterial soaps and wipes. Ethanol kills organisms by denaturing their proteins and dissolving their lipids and is effective against most bacteria and fungi, and many viruses, but is ineffective against bacterial spores.
- Solvent: As a good solvent, you will find ethanol frequently used in many medicine, such as cough syrups.
- Drug: For therapeutic neurolysis: It is injected proximate to nerve tissues and into spinal subarachnoid spaces to produce degeneration of nerve function (neurolysis) for control of chronic pain.
Poison
A couple of days ago I heard in the news that Amy Winehouse‘s death this summer was the result of alcohol poisoning….
A pathologist told a coroner’s court in north London that alcohol toxicity was the cause of the 27-year-old’s death, with her blood-alcohol levels measured at more than five times the legal limit for driving.
The report said that Amy’s blood alcohol content was 416 mg per decilitre at the time of her death. That’s 0.416% alcohol in the blood and about 9 drinks for a woman 45 kg like Amy. When large amounts of alcohol are consumed in a short period of time, alcohol poisoning can occur. Above a blood alcohol concentration of 0.5%, alcohol depresses nerves that control involuntary actions such as breathing and cardiovascular regulation. Moreover, it can cause hypothermia (low body temperature), hypoglycemia (too little blood sugar) and sever dehydration. These effects can lead to cause seizures, permanent brain damage, and ultimately death. That’s why you should never underestimate the effects of alcohol. And think twice before sipping more than three drinks in a row! Better to be safe than sorry.
Posted on November 22, 2011, in chemistry in everyday life, food, materials, science facts and tagged alcohol, Amy Winehouse, drink, ethanol. Bookmark the permalink. 17 Comments.
Comment from a reader on Facebook:
“Our European ancestors were purifying water with alcohol, while the Asian people were boiling it instead for disinfection. Therefore, there was evolutionary pressure in European populations to be able to process alcohol, which is absent in Asians.
This might be part of the explanation why Alcohol dehydrogenase is less efficient in Asian populations.”
This explanation was part of an Evolutionary Biology course. You can find a relevant link here: http://www.readersread.com/excerpts/survivalofthesickest.htm
Today i spent 300 $ for platinium roulette system ,
i hope that i will earn my first $$$ online
This is my first time go to see at here and i am truly happy to read all
at single place.
Really good
indeed must
also here can be appear other as observed prof dr mircea orasanu for poisson
in more situations repeated appear some observed by prof dr mircea orasanu as an art
situations can be considered for many geometrical transforms as observed by prof dr mircea orasanu in case of prof dr doc stefan I.gheorghita prof hollinger or prof nicolae dinculeanu with mathematical analysis or prof stefan zarea 1968 from polytechnic inst
these are considered as observed prof dr mircea orasanu lead to followed for thus and sure calugarita and arghiriade or polubarinova kochina , v smirnov must development in more situations , but no marcel chirita
we observed that there are still many properties that must mentioned ,and for acohol tnat is very important and application of mathematical application we mention on emerich toth that love greek geometry and together ene horia, marcel chirita , arghiriade , calugarita and l panaitopol have discovered pytagoras theorem and equation gr II ,and also caius iacob with lazar dragos
in many situations we questioned certain aspects in CHEMISTRY as observed prof dr mircea orasanu in followed and
APPLICATION OF ANALYTICAL MECHANICS IN CHEMISTRY
Making these substitutions into (5) gives
Each term now contains an expression of the form r(r/s), which can also be written as (r2/2)/s, so the overall expression can be re-written as
The quantity inside the square brackets is simply the kinetic energy, conventionally denoted by T. Thus the generalized force FX, and similarly the generalized force FY, can be expressed as
These are the Euler-Lagrange equations of motion, which are equivalent to Newton’s laws of motion. (Notice that if X is identified with x in equation (5), then FX reduces to Newton’s expression for fx, and likewise for the other components.)
If the total energy is conserved, then the work done on the particle must be converted to potential energy, conventionally denoted by V, which must be purely a function of the spatial coordinates x,y,z, or equivalently a function of the generalized configuration coordinates X,Y, and possibly the derivatives of these coordinates, but independent of the time t. (The independence of the Lagrangian with respect to the time coordinate for a process in which energy is conserved is an example of Noether’s theorem, which asserts that any conserved quantity, such as energy, corresponds to a symmetry, i.e., the independence of a system with respect to a particular variable, such as time.) If the potential depends on the derivatives of the position coordinates it is said to be a velocity-dependent potential, as discussed in the note on Gerber’s Gravity. However, most potentials depend only on the position coordinates and not on their derivatives. In that case we have
Comparing this with equation (2), we see that
and therefore the Euler-Lagrange equations (6) for conservative systems can be written as
Rearranging terms, we have
Furthermore, since V is purely a function of the configuration variables, independent of their rates of change, we can just as well substitute (TV) in place of T on the right sides of these equations, so in terms of the parameter L = T V these equations can be written simply as
The quantity L is called the Lagrangian. This derivation was carried out for a single particle moving with two degrees of freedom in three-dimensional space, but the same derivation can be applied to collections of any number of particles. For a set of N particles there are 3N configuration coordinates, but the degrees of freedom will often be much less, especially if the particles form rigid bodies. Letting q1, q2, .., qn denote a set of generalized configuration coordinates for a conservative physical system with n degrees of freedom, the equations of motion of the system are
where L is the Lagrangian of the system, i.e., the difference between the kinetic and the potential energies, expressed in terms of the generalized coordinates and their time derivatives. These equations are usually credited jointly to Euler along with Lagrange, because although Lagrange was the first to formulate them specifically as the equations of motion, they were previously derived by Euler as the conditions under which a point passes from one specified place and time to another in such a way that the integral of a given function L with respect to time is stationary. (Roughly speaking, “stationary” means that the value of the integral does not change for incremental variations in the path.) This is a fundamental result in the calculus of variations, and can be applied to fairly arbitrary functions L (i.e., not necessarily the Lagrangian). For a derivation of the Euler conditions, see Relatively Straight.
To illustrate the application of these equations, consider a simple mass-spring system, consisting of a particle of mass m on the x axis attached to the end of a massless spring with spring constant k and null point at x = 0. For any position x, the spring exerts a force equal to F = kx, and the potential energy is the integral of force with respect to displacement. Similarly the kinetic energy is the integral of the inertial force F = ma with respect to displacement. Thus the kinetic and potential energies of the system are
Therefore the Lagrangian of the system is
The partial derivatives are
Substituting into Lagrange’s equation, we get the familiar equation of harmonic motion for a mass-spring system
Of course, this simply expresses Newton’s second law, F = ma, for the particle. It’s also equivalent to the fact that the total energy E = T + V is constant, as can be seen by differentiating E with respect to t and then dividing through by dx/dt.
The equivalence between the Lagrangian equation of motion (for conservative systems) and the conservation of energy is a general consequence of the fact that the kinetic energy of a particle is strictly proportional to the square of the particle’s velocity. Of course, in terms of the generalized parameters, it’s possible for the kinetic energy to be a function of both q and (see, for example, Time for a Rocking Chair), but since the transformation dx = (x/q)dq between x and q is equivalent to dx/dt = (x/q)dq/dt, it follows that for a fixed configuration the kinetic energy is proportional to the squares of the generalized velocity parameters. Therefore, in general, we have
where we’ve made use of the fact that the potential energy V (for conservative systems) is independent of . Now, the total energy is E = T + V = 2T L, so the conservation of energy can be expressed in the form
The two terms on the right hand side can be expanded as
Substituting into the previous equation and dividing through by (applying analytic continuation to remove the singularity when = 0), we see that the conservation of energy implies
which is just Lagrange’s equation of motion. Of course, the same derivation applies to any number of particles, and their generalized coordinates.
The correspondence between the conservation of energy and the Lagrangian equations of motion suggests that there might be a convenient variational formulation of mechanics in terms of the total energy E = T + V (as opposed to the Lagrangian L = T V). Notice that the partial derivative of L with respect to x’ is the momentum of the particle. In general, given the Lagrangian, we can define the generalized momenta as
(The partial of V is zero, so it’s inclusion and sign in this definition is a matter of convention.) Thus to each generalized configuration coordinate qj there corresponds a generalized momenta pj. In our simple mass-spring example with the single generalized coordinate q = x, the total energy H = T + V in terms of these conjugate parameters is
The function H(q,p) is called the Hamiltonian of the system. Taking the partial derivatives of H with respect to p and q, we have
Notice that, in this example, p/m equals q’ (essentially by definition, since p = mv), and kq equals -p’ (by the equation of motion). In general it can be shown that, for any conservative system with generalized coordinates qj and the corresponding momenta pj, if we express the total energy H in terms of the qj and pj, then we have
These are Hamilton’s equations of motion. Although they are strictly equivalent to Lagrange’s and Newton’s equations, the equations of Hamilton have proven to be more suitable for adaptation to quantum mechanics. The Lagrangian and Hamiltonian formulations of mechanics are also notable for the fact that they express the laws of mechanics without reference to any particular coordinate system for the configuration space. Of course, in their original forms, they assumed an absolute time coordinate and perfectly rigid bodies, but with suitable restrictions they can be adapted to relativistic mechanics as well.
In quantum mechanics, a pair of conjugate variables qj, pj, such as position and momentum, generally do not commute, which means that the operation consisting of a measurement of qj followed by a measurement of pj is different than the operation of performing these measurements in the reverse order. This is because the eigenstates corresponding to the respective measurement operators are incompatible. As a result, the system cannot simultaneously have both a definite value of qj and a definite value of pj. See Fourier Transforms and Uncertainty for more on this topic.
Pentru a se obţine ulterior o formă convenabila se înlocuieşte D = C0/2 Þ u0(x,y) = ½ C0x.
c) Dacă m > 0 , m = l2(l>0) se obţine
X” = l2X Þ X = A×cosh lx + B×sinh lx
Trebuie determinat coeficientul C astfel încât funcţia definită de această serie să se reducă la o anumită distribuţie de temperaturi f(y) pentru x = 1.Aşadar trebuie ca
O bară de lungime l are suprafaţa laterală perfect izolată şi este atât de subţire încât fluxul de căldură poate fi considerat unidimensional. Temperatura iniţială este de 100 ºC în întreaga bară. La momentul t = 0, temperatura capătului din stânga al barei este redusă brusc la 50 ºC şi menţinută la această temperatură în timp ce temperatura capătului din dreapta este menţinută la 100 ºC. Să se determine temperatura în orice punct al barei pentru orice moment de timp ulterior.
Rezolvare
Este necesar rezolvarea ecuaţiei unidimensionale a căldurii: , cu condiţia la limită u(0,t) = 50 şi u(l,t) = 100 şi condiţia iniţială u(x,0) = 100.
Dacă se presupune o soluţie de tipul unui produs u(x,t) = X(x)T(t), prin diferenţiere ,înlocuire şi împărţire la XT se obţine:
X = A×cosh lx + B×sinh lx şi T = C×exp(l2t/a2) (2.42)
Iar u(x,t) = X(x)T(t) = (A×cosh(lx)+B×sinh(lx))(C×exp(l2t/a2))
Această soluţie trebuie respinsă imediat deoarece datorită funcţiei exponenţiale la puterea pozitiva a lui t rezultă că temperatura creşte fără limită dacă t®¥, lucru imposibil în condiţiile date de problemă
O bară cilindrică subţire de lungime l are suprafaţa curbată perfect izolată termic. Capătul din stânga este menţinut la temperatura constantă u = 0, iar cel din dreapta radiază liber în aer la temperatura constantă u = 0. Distribuţia iniţială a temperaturii în bară este: u(x,0) = f(x). Să se determine temperatura în bară la orice moment de timp ulterior.
Rezolvare
Deoarece bara este foarte subţire iar suprafaţa laterală este perfect izolată, se presupune că în orice punct al unei secţiuni transversale temperatura este constantă şi că fluxul de căldură prin bară se transmite în întregime pe direcţia x
Pentru capătul din stânga avem condiţia la limită u(0,t) = 0. Pentru capătul din dreapta avem transfer de căldură prin radiaţie, pentru care trebuie formulată o expresie analitică a soluţiei înainte de a trece la rezolvarea propriu zisă a problemei. Conform legii lui Stefan cantitatea de căldură radiată este:
dQ = s(T4 – Ta4)dS dT
Se presupune că soluţia este de tipul unui produs de forma:
u(x,t) = X(x)×T(t)
Prin separarea variabilelo
These are the Euler-Lagrange equations of motion, which are equivalent to Newton’s laws of motion. (Notice that if X is identified with x in equation (5), then FX reduces to Newton’s expression for fx, and likewise for the other components.)
If the total energy is conserved, then the work done on the particle must be converted to potential energy, conventionally denoted by V, which must be purely a function of the spatial coordinates x,y,z, or equivalently a function of the generalized configuration coordinates X,Y, and possibly the derivatives of these coordinates, but independent of the time t. (The independence of the Lagrangian with respect to the time coordinate for a process in which energy is conserved is an example of Noether’s theorem, which asserts that any conserved quantity, such as energy, corresponds to a symmetry, i.e., the independence of a system with respect to a particular variable, such as time.) If the potential depends on the derivatives of the position coordinates it is said to be a velocity-dependent potential, as discussed in the note on Gerber’s Gravity. However, most potentials depend only on the position coordinates and not on their derivatives. In that case we have
Furthermore, since V is purely a function of the configuration variables, independent of their rates of change, we can just as well substitute (TV) in place of T on the right sides of these equations, so in terms of the parameter L = T V these equations can be written simply as
The quantity L is called the Lagrangian. This derivation was carried out for a single particle moving with two degrees of freedom in three-dimensional space, but the same derivation can be applied to collections of any number of particles. For a set of N particles there are 3N configuration coordinates, but the degrees of freedom will often be much less, especially if the particles form rigid bodies. Letting q1, q2, .., qn denote a set of generalized configuration coordinates for a conservative physical system with n degrees of freedom, the equations of motion of the system are
in problem of alcohol that is very important must used more and many considerations of P. Fermat , or Meusnier theorem or Egregium Theorem due of Gauss as observed prof dr mircea orasanu for critical or fundamental points of alcohol fluids ,then appear and other situations Can it not be the case that teachers of mathematics can do both? Is there not a case to be made for considering the contribution teachers of mathematics might make in terms of citizenship and plan geometry or spatial geometry ?
Set against this background it is not surprising that the debate around issues of social justice and equal opportunities in the classroom came to whither on the vine during the last decade in more . Further it is not surprising that schools are now seen
obviously there are important aspects in alcohol not only chemical but physically as can be about prof dr mircea orasanu and prof drd horia orasanu as followed in
NONHOLONOMIC OPTIMIZATION IN ANALYTICAL MECHANICS ,thus
ABSTRACT
Basic form of representation.
In some systems, all objects are described as polygon surfaces.
For example, Inventor.
Polygons are easy to process, so rendering and display of objects is sped up. Thus, some systems allow objects to be described in other ways (such as splines), but reduce all objects to polygons for processing.
1 INTRODUCTION
We specify objects as a set of vertices and associated attributes.
This information can be stored in tables, of which there are two types: geometric tables and attribute tables.
The geometry can be stored as three tables: a vertex table, an edge table, and a polygon table. Each entry in the vertex table is a list of coordinates defining that point. Each entry in the edge table consists of a pointer to each endpoint of that edge. And the entries in the polygon table define a polygon by providing pointers to the edges that make up the polygon.
We can eliminate the edge table by letting the polygon table reference the vertices directly, but we can run into problems, such as drawing some edges twice, because we don’t realize that we have visited the same set of points before, in a different polygon. We could go even further and eliminate the vertex table by listing all the coordinates explicitly in the polygon table, but this wastes space because the same points appear in the polygon table several times.
Using all three tables also allows for certain kinds of error checking. We can confirm that each polygon is closed, that each point in the vertex table is used in the edge table and that each edge is used in the polygon table.
Tables also allow us to store additional information. Each entry in the edge table could have a pointer back to the polygons that make use of it. This would allow for quick look-up of those edges which are shared between polygons. We could also store the slope of each edge or the bounding box for each polygon–values which are repeatedly used in rendering and so would be handy to have stored with the data.
as is usual can be meet many aspects of science and therefore aa can be consider prof dr mircea orasanu problem of conformal transform can be known bt dr d homentkovsky although has been associated with caius jacob and thus these can be nor insight for problems great addition for the student to see where the triangle is formed on the fig.1 by angle theta. That being said, a student would only need to have an angle and a side and would thus be able to produce sides and angles associated with that triangle. This too would create a stronger understanding of how trigonometric functions are a proportion of arc length in radians, and in turn, give the student the ability to apply this concept to both circles and triangles
,
in many situations are observed as prof dr mircea orasanu and prof drd horia orasanu as continued
in thus with many