How is coffee decaffeinated?
Apparently it’s National Coffee Day today, read below to learn in less than a page how coffee gets decaffeinated – not an easy task!
Coffee contains over 400 chemicals important to the taste and aroma of the final drink: it is therefore challenging to remove only caffeine while leaving the other chemicals at their original concentrations.
 To get rid of the caffeine, unroasted (green) beans are at first steamed. The beans are then rinsed with a “solvent” chemical that extracts the caffeine while leaving the other essential chemicals in the coffee beans. The process is repeated anywhere from 8 to 12 times until caffeine is removed in 97%-99.9%. So what is this magic “solvent” chemical. which is able to selectively remove caffeine while preserving the coffee aroma?
To get rid of the caffeine, unroasted (green) beans are at first steamed. The beans are then rinsed with a “solvent” chemical that extracts the caffeine while leaving the other essential chemicals in the coffee beans. The process is repeated anywhere from 8 to 12 times until caffeine is removed in 97%-99.9%. So what is this magic “solvent” chemical. which is able to selectively remove caffeine while preserving the coffee aroma?
This solvent is nothing else but carbon dioxide (CO2), which is also found in our very own atmosphere. However, as you might have guessed, CO2 is not used for decaffeination as a gas from the atmosphere. For the decaffeination process, the coffee beans are soaked in a special form of CO2 called “supercritical” CO2. OK, so now you naturally have to ask what the heck is this “supercritical” CO2??
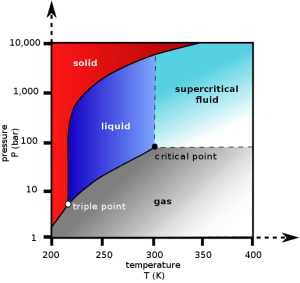
Every substance (water, hydrogen, oxygen, etc) has a so-called “critical point”. The critical point is the point above which, the distinction between liquid and gas phases of that substance stops being meaningful. That is you cannot tell any more whether this substance is gas or liquid. This new “phase”, being neither gas or liquid is called “supercritical”. A “supercritical fluid” can effuse through solids like a gas, and dissolve materials like a liquid.
So how does supercritical CO2 look like?
To achieve the supercritical state for most substances requires extreme temperatures and pressures. Carbon dioxide, however, has a fairly accessible critical point at ~ 31.1°C and 73 atm (our atmosphere has 1 atm). And it seems that supercritical CO2 has unique properties such that it can selectively extract caffeine from coffee beans, producing decaf coffee.
Carbon dioxide pressure-temperature phase diagram. The critical point is found at T=31.1°C and 73 atm. Above the critical point, one cannot really tell whether CO2 is liquid or gas. This new phase is called “supercritical”.
Posted on September 29, 2011, in chemistry in everyday life, food, materials and tagged food processing, phase diagram, supercritical fluids. Bookmark the permalink. 3 Comments.
(16) How to Eat, Move and be Healthy, page 221,
Paul Chek. It can also keep you awake which is why you need to avoid it before bedtime.
Regular vegetable oil works great, but some prefer a
more professional approach.
Excellent goods from you, man. I’ve bear in mind your stuff previous to and you are just extremely magnificent. I really like what you have bought right here, certainly like what you are saying and the best way during which you are saying it. You’re making it enjoyable and you continue to care for to keep it sensible. I can’t wait to learn much more from you. This is actually a tremendous website.|
Pingback: Understanding the Weird History of Decaffeinated Coffee and How It’s Made – Compass Coffee